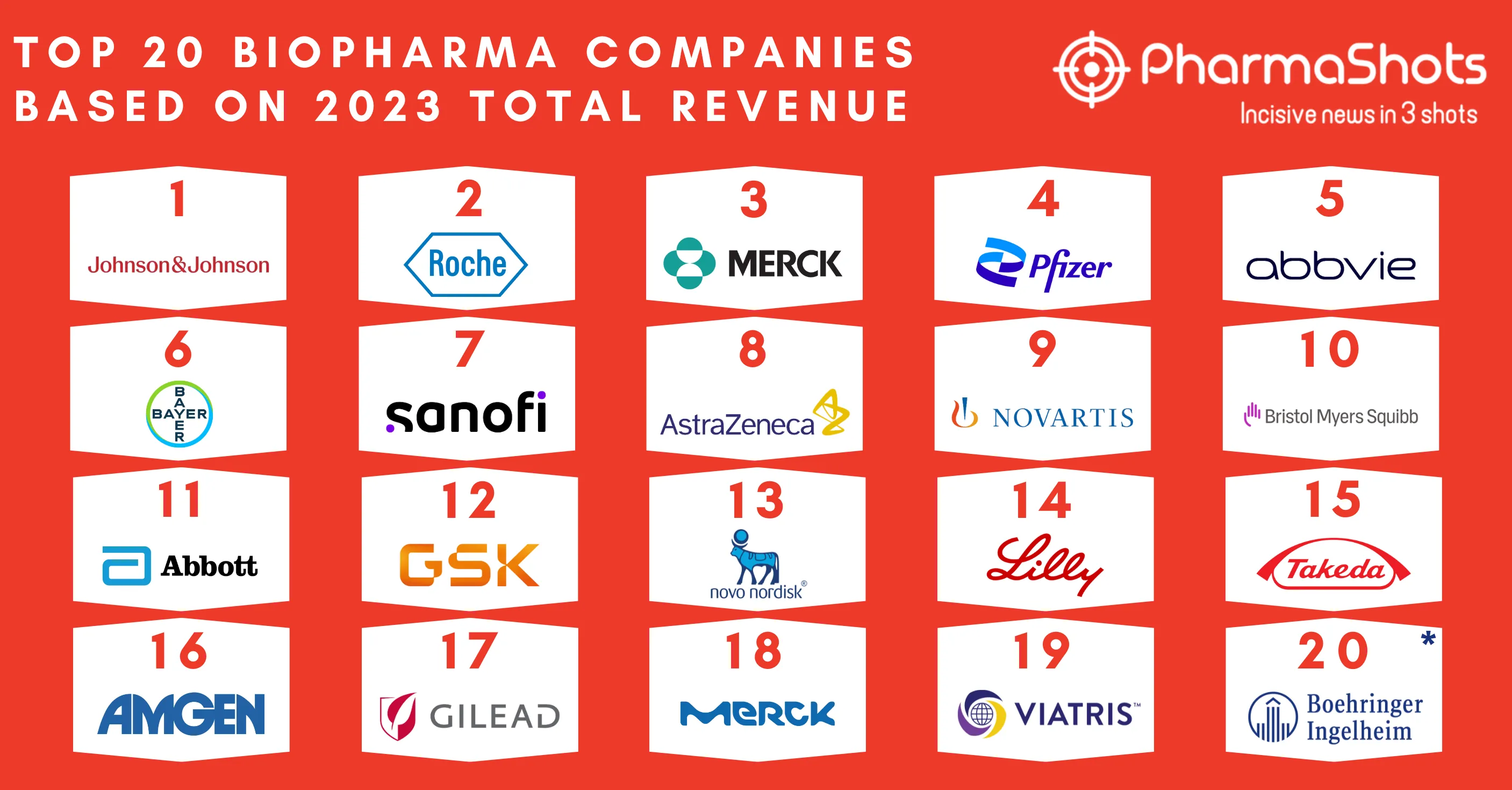
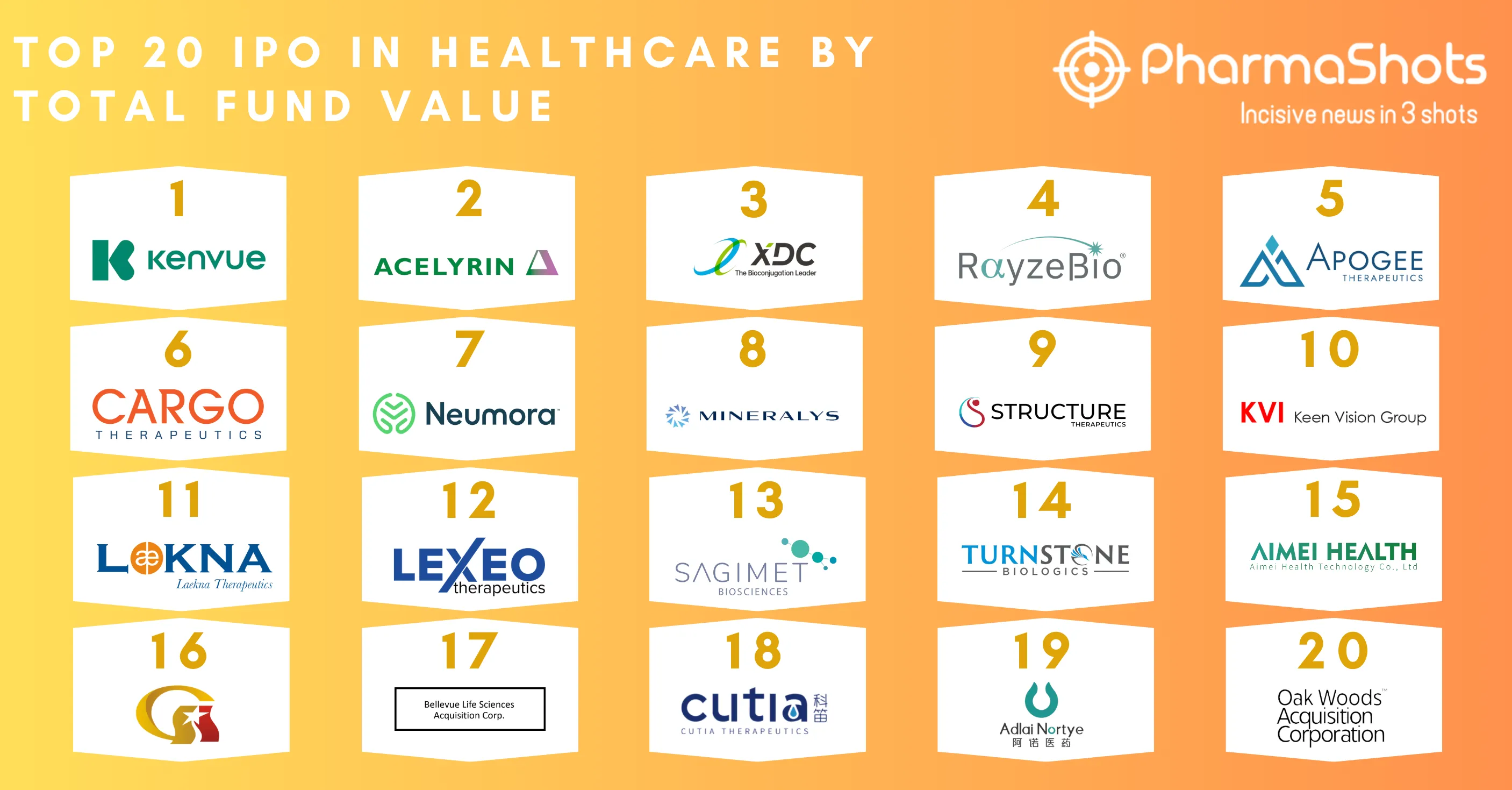
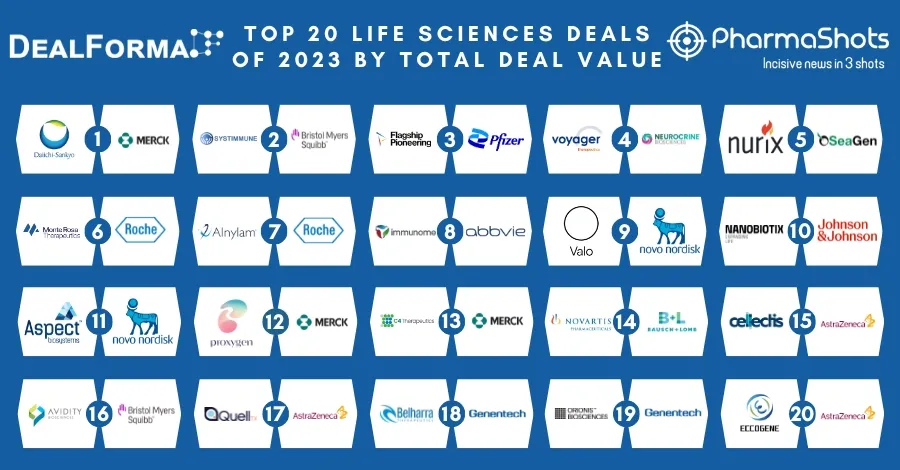
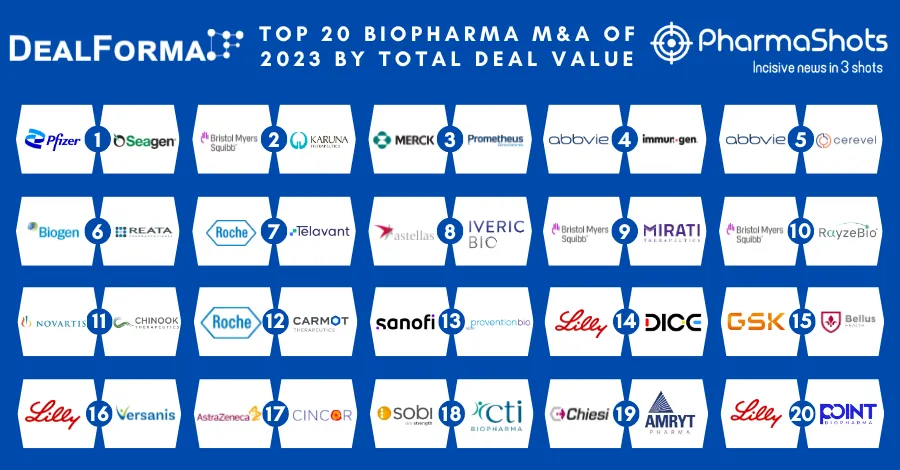
20 Contract Development and Manufacturing Organizations (CDMOs) of 2022
Shots:
- Over the years, CDMOs have significantly transformed the course of pharmaceutical and biopharmaceutical companies by leveraging their flexible manufacturing facilities and on-time drug deliveries
- CDMOs help meet the ever-evolving needs of the healthcare sector by fostering the use of new-age technologies and advanced drug development and manufacturing processes
- PharmaShots brings a condensed report on 20 Contract Development Manufacturing Organizations (CDMOs), arranged alphabetically, that remained the talk of the town in 2022

20. Aenova
Founded Year: 1949
Total Employees: ~4,000
Headquarters: Munich, Germany
- Aenova Group is a leading CDMO that spans the entire development and production value chain for various dosage forms (solid, semi-solid, liquid, sterile, and non-sterile) in drugs and food supplements for human and animal health
- Aenova provides comprehensive services encompassing product development, raw material procurement, production, analytics, market release, packaging, and logistics
- With operations in 15 locations across Germany, Switzerland, Italy, Ireland, Romania, and the USA, Aenova is involved in the development, production, and packaging of pharmaceuticals.

19. AGC Biologics
Founded Year: 2018
Total Employees: ~2,500
Headquarters: Washington, United States
- Earlier in 2016, all the shares of CMC Biologics were acquired by AGC Asahi Glass (AGC), a Japan-based manufacturer of glass, chemicals, and high-tech materials. As a result of this acquisition, in Jan 2018, AGC Biologics was formed as a CDMO led by the convergence of Asahi Glass Company (AGC) Bioscience, Biomeva GmbH (Heidelberg, Germany), and CMC Biologics
- The company's primary goal is to address early-phase, late-phase, and commercial production demands at small and big scales by offering microbial and mammalian capabilities
- AGC Biologics is indulged in the development and manufacturing of mammalian and microbial-based therapeutic proteins, plasmid DNA (pDNA), messenger RNA (mRNA), viral vectors, and genetically engineered cell

18. Avid Bioservices
Founded Year: 2002
Total Employees: ~350
Headquarters: California, United States
- In 1993, Avid embarked on a journey as a biologics development and manufacturing entity under the name Peregrine Pharmaceuticals
- By 2002, Avid transitioned into a wholly owned subsidiary of Peregrine Pharmaceuticals. The year 2018 saw Avid Bioservices Inc. emerging as an independent biologics manufacturer listed on NASDAQ (NASDAQ: CDMO)
- The company is focused on the specialized development and production of monoclonal antibodies and recombinant proteins, providing services such as process development, analytical development, and cGMP manufacturing

17. Boehringer Ingelheim
Founded Year: 1998
Total Employees: ~5,000
Headquarters: Ingelheim, Germany
- Boehringer Ingelheim BioXcellence is an integral part of the biopharma giant Boehringer Ingelheim. It was established in the year 1998 as a contract manufacturing business
- This biotech firm was laid on the foundation of Berofor (interferon alpha), the company’s first biologic to be launched in the market. Today, the company has expanded its business into contract manufacturing by adding global capabilities in mammalian cell culture, microbial fermentation, and GMP
- The company has four biopharmaceutical manufacturing facilities across the globe i.e. Biberach (Germany), Vienna (Austria), Fremont (USA), and Shanghai (China). BioXcellence is also adamant about providing products tailored to meet specific customer requirements at any phase in the process

16. Cambrex
Founded Year: 1981
Total Employees: ~2,000
Headquarters: New Jersey, United States
- A renowned CDMO, Cambrex Corp, is dedicated to the development, testing, and building of active pharmaceutical ingredients. Additionally, the company also provides drug substances, drug products, and analytical services across the entire drug lifecycle
- Along with its presence in the development of branded products, the company indulges in the business of developing and manufacturing API and dosage forms for the generic market
- The company mainly provides drug substance technologies and capabilities, including biocatalysis, continuous flow, controlled substances, solid-state science, material characterization, and highly potent application programming interfaces among others. Cambrex has its cGMP manufacturing facilities across the globe with areas, including the United States and Europe

15. Catalent
Founded Year: 2007
Total Employees: ~18,000
Headquarters: New Jersey, United States
- Catalent is a multinational corporation based in the United States. The company provides delivery technologies, development, drug manufacturing, biologics, gene therapies, and consumer health products. Developed as a private firm in 2007, Catalent went public in 2019 and generated a revenue of $4.82B in 2022
- The company operates under four segments, which include, Development and Clinical Services, Oral Technologies, Sterile Technologies, and Packaging Services. The Development and Clinical Services segment provides manufacturing, packaging, storage, and inventory management for clinical trials. The Oral Technologies segment offers formulation, development, and manufacturing services for oral dose forms
- The Sterile Technologies segment provides proprietary and traditional dose forms for different administration routes, whereas the Packaging Services segment offers packaging services for pharmaceuticals, biologics, consumer health, and veterinary products

14. CordenPharma
Founded Year: 2006
Total Employees: ~3,000
Headquarters: Plankstadt, Germany
- CordenPharma is a CDMO that focuses on providing prominent biotech and pharmaceutical firms with advanced techniques to accelerate their drug development lifecycle
- The company invests in developing APIs, Excipients, Drug Products, and associated packaging services. Corden provides an end-to-end service with a strategic focus on Small Molecules, Peptides, Oligonucleotides, customized Lipid Excipients, Lipid NanoParticles, and Sterile Injectables
- Corden has a widely spread business with 12 facilities across Europe and North America. The company generated a total revenue of $933.7M in 2022

13. Emergent BioSolutions
Founded Year: 1998
Total Employees: ~2,500
Headquarters: Maryland, United States
- An integral part of Emergent BioSolutions, Emergent Bioservices is a renowned name among the CDMOs. Emergent BioSolutions is a company indulged in the business of developing vaccines and antibodies for infectious diseases and opioid overdoses and also provides medical devices for biodefense purpose
- Emergent Bioservices has established experience in scientific and regulatory compliance and provides services across the development and manufacturing of resources required by major pharma and biotech companies
- The company has efficient technology transfer capabilities. Emergent Bioservices expands its business by serving companies needing small volumes of materials for clinical trials or large-scale manufacturing for commercial products

12. Eurofins
Founded Year: 1987
Total Employees: ~61,000
Headquarters: Van Fleuri, Luxembourg
- Eurofins Scientific has spread its business into the fields of food, environment, pharmaceutical, cosmetic product testing, discovery pharmacology, forensics, advanced material sciences, agriscience, Contract Research Services, genomics, clinical study support, and BioPharma Contract Development and Manufacturing
- The company has marked its position among the major CDMOs as it provides an integrated end-to-end solution for preclinical and clinical outsourcing services of both drug substances or APIs or drug products for the development of biologics or small molecules
- Eurofins combines its expertise in both small molecule and biologics with scale-up knowledge to serve major pharma and biotech companies

11. Fareva
Founded Year: 1981
Total Employees: ~13,000
Headquarters: Gare, Luxembourg
- A France-based contract development manufacturing organization, Fareva is a renowned name amongst the CDMOs. In 2020, Fareva acquired Pierre Fabre, an oncology injectable plant and a monoclonal antibody production facility as a part of its expansion program
- With 11 manufacturing plants across France, Fareva generates more than 650M euros annually. Fareva operates in more than 80 industrial sites and offers biopharma companies solids, semi-liquids, semi-solids, and sterile liquids products among others
- Over the years Fareva has been known for partnering with several pharmaceutical and biopharma companies to meet their growing needs with their advanced production and development facility

10. Fujifilm Diosynth
Founded Year: 1923
Total Employees: ~2,500
Headquarters: Texas, United States
- Fujifilm Diosynth is a renowned CDMO dedicated to the development and manufacturing of biologics, vaccines, and advanced therapies. Diosynth became an integral part of Fujifilm in 2011
- With headquarters situated in the United States, Diosynth has manufacturing facilities across Morrisville and North Carolina in the United States and Hillerod, Denmark in Europe among others
- Moreover, the company offers complete solutions in pharmaceutical manufacturing from pre-clinical investigation and process development to commercial cGMP production

9. Lonza
Founded Year: 1923
Total Employees: ~2,500
Headquarters: Texas, United States
- Based in Switzerland, Lonza combines technological innovation with pharmaceutical manufacturing. The company has collaborated with various companies in the pharmaceutical, biotech, and nutritional markets
- From early-phase research to custom development and production of active pharmaceutical ingredients to innovative dosage forms for the pharma, consumer health, and nutrition industries, Lonza offers a wide range of services and products
- In 2022, the company was indulged into more than 825 preclinical and clinical small/large molecules, more than 195 small/large molecules, and developed ~250B capsules. Moreover, in 2022, Lonza generated a revenue of $6.7B

8. MilliporeSigma
Founded Year: 1954
Total Employees: ~19,000
Headquarters: Massachusetts, United States
- MilliporeSigma is an integral part of Merck KGaA, which works as a CDMO and provides services to the life science and pharmaceutical industry. MilliporeSigma was formed by Merck’s acquisition of Millipore Corporation in 2010 and its acquisition of Sigma-Aldrich in 2015
- MilliporeSigma provides services ranging from preparation, separation, filtration, and monitoring. The company has a portfolio consisting of Cell Culture Sample Preparation, Chromatography Sample Preparation, Protein Sample Preparation, Lab Scale Filters, and Large-Scale Sterile Filtrations among others
- The company specializes in providing products and services in their specific life science business, including Research Solutions, Process Solutions, and Applied Solutions. It collaborated with the global scientific community to make research and biotech production a hassle-free process

7. Patheon
Founded Year: 1974
Total Employees: ~10,000
Headquarters: Massachusetts, United States
- In 2017, Thermo Fisher Scientific acquired a renowned CDMO, Patheon for ~7.2B. Today, Thermo Fisher provides pharma services solutions for drug development, clinical trial logistics, and commercial manufacturing to customers through its acquired asset Patheon
- The company serves more than 60 locations across the globe with its manufacturing sites situated in areas, including the United States, Europe, Australia, and major parts of Asia. Patheon delivers services across all phases of development, including API, biologics, viral vectors, cGMP plasmids, formulations, clinical trial solutions, and logistic services along with commercial manufacturing and packaging
- The company also indulges in the business of providing tailored requirements for drug development through its Patheon Quick to Care and Patheon Quick to Clinic programs. Today, Patheon serves a wide range of pharmaceutical and biotech companies

6. Piramal
Founded Year: 1984
Total Employees: ~12,000
Headquarters: Mumbai, India
- Ranging from drug discovery and development to manufacturing and antibody drug conjugation, Piramal Pharma Solutions is a well-established CDMO. The company operates profusely in North America, Europe, and Asia
- A global leader in developing and manufacturing APIs, Piramal Pharma Solutions develops and manufactures biologics, including vaccines, mABs, and gene therapies through its investment in Yapan Bio Private Limited
- The company operates in 30 countries and has a global presence in over 100 markets, making it a potential partner company for pharmaceutical and biopharma companies

5. Recipharm
Founded Year: 1995
Total Employees: ~9000
Headquarters: Stockholm, Sweden
- Recipharm started its journey with a single solid dose facility in Stockholm and now has grown to have 30+ facilities through acquisition and organic growth across Europe, India, Israel, and North America
- The company's services cover the entire life cycle of a pharmaceutical product, from drug substance to commercial manufacturing, thereby enabling clients to get products to market in a timely and cost-efficient way
- Recipharm focuses on supporting pharmaceutical companies by providing services that take products from early development through to commercial productions

4. Rentschler
Founded Year: 1927
Total Employees: ~1,000
Headquarters: Laupheim, Germany
- Rentschler Biopharma SE has a distinguished presence in the CDMO community. A family-owned company based in Germany, Rentschler develops and manufactures biopharmaceuticals. The company has worked in diverse domains, including project management, regulatory support, and consulting
- The company specializes in drug production, bioprocess development, and manufacturing. With expertise with more than 300 molecules and over 110 different therapeutic protein formats, Rentschler works steadfastly for the availability of biopharmaceuticals
- The company signs strategic partnerships with leading biopharma companies to meet their development and manufacturing needs. Recently the company’s ATMP facility in the UK received MHRA approval

3. Samsung Biologics
Founded Year: 2011
Total Employees: ~4,400
Headquarters: Incheon, Republic of Korea
- The biotech branch of Samsung Group is called Samsung Biologics. Samsung Biologics offers cell line development, final aseptic fill/finish as well as laboratory testing services at every stage of biopharmaceutical product development and manufacturing
- The company currently has four manufacturing plants, holding a total capacity of 604KL. The company has partnered with several pharmaceutical companies such as Pfizer, GlaxoSmithKline, Eli Lilly, and AstraZeneca for various products
- Samsung Biologics also partnered with Moderna for fill-finish, packaging, and labeling of its mRNA vaccine, Spikevax, during the COVID-19 pandemic

2. Siegfried
Founded Year: 1873
Total Employees: ~3,600
Headquarters: Zofingen, Switzerland
- Siegfried is a leading CDMO with a global network of 12 production sites in 7 countries across three continents. The company offers contract development and manufacturing services for active pharmaceutical ingredients, intermediates, and finished dosage forms.
- Siegfried provides a comprehensive range of services, from process development and optimization to registration, production, packaging, and logistics under a single roof
- The company broadly offers complex oral, sterile, ophthalmic, and inhalation formulations as well as viral vectors and associated technologies for custom development and manufacturing as well as licensing

1. Wuxi AppTech
Founded Year: 2000
Total Employees: ~44,300
Headquarters: Shanghai, China
- Wuxi AppTec provides a broad portfolio of R&D, drug testing, and manufacturing services across Asia, Europe, and North America. Its end-to-end services include chemistry drug CRDMO (Contract Research, Development, and Manufacturing Organization), biology discovery, preclinical testing and clinical research services, and cell and gene therapies CTDMO (Contract Testing, Development, and Manufacturing Organization)
- The company offers a CRDMO Platform for Small molecules, Oligonucleotides, and Peptides. As a CTDMO, Wuxi provides comprehensive services for advanced biopharmaceuticals such as genes, cells, and others
- Its biopharmaceuticals and medical device testing services span the entire product lifecycle from IND applications to commercial Lot Release for Advanced Therapies, Proteins, and Vaccines
Related Post: Top 20 Countries in Healthcare Innovation
Tags

Shivani is a content writer at PharmaShots. She has a keen interest in recent innovations in the life sciences industry. She covers news related to Product approvals, clinical trial results, and updates. She can be contacted at connect@pharmashots.com.